Source: YouTube
The Chemistry of Lead Acid Batteries
Overview of Lead Acid Batteries
A lead acid battery consists of a negative electrode made of spongy or porous lead, a positive electrode made of lead oxide, and an electrolytic solution of sulfuric acid and water. The electrodes are separated by a chemically permeable membrane to prevent electrical shorting.
Chemical Reactions in Lead Acid Batteries
Lead acid batteries store energy through reversible chemical reactions. During discharge, lead sulfate crystals form at the electrodes, releasing electrons. The electrolyte becomes less concentrated as sulfate is used in the formation of lead sulfate.
Charging Process
During charging, lead sulfate is converted back to lead and lead oxide at the electrodes. Hydrogen gas is evolved as a by-product, leading to the “gassing” of the battery. Gassing poses safety risks and reduces water levels in the battery.
Impact of Gassing
Gassing can cause safety concerns, water loss, and active material shedding, reducing battery capacity. To prevent these issues, charging should not exceed the voltage that causes gassing.
Lead Sulfate Formation
Lead sulfate formation on the electrodes affects the battery’s discharge efficiency. The morphology of lead sulfate crystals influences the battery’s performance and rechargeability.
Lead acid batteries are widely used for various applications due to their reliability and cost-effectiveness. Understanding the chemical processes occurring within these batteries is crucial for optimizing their performance and longevity.
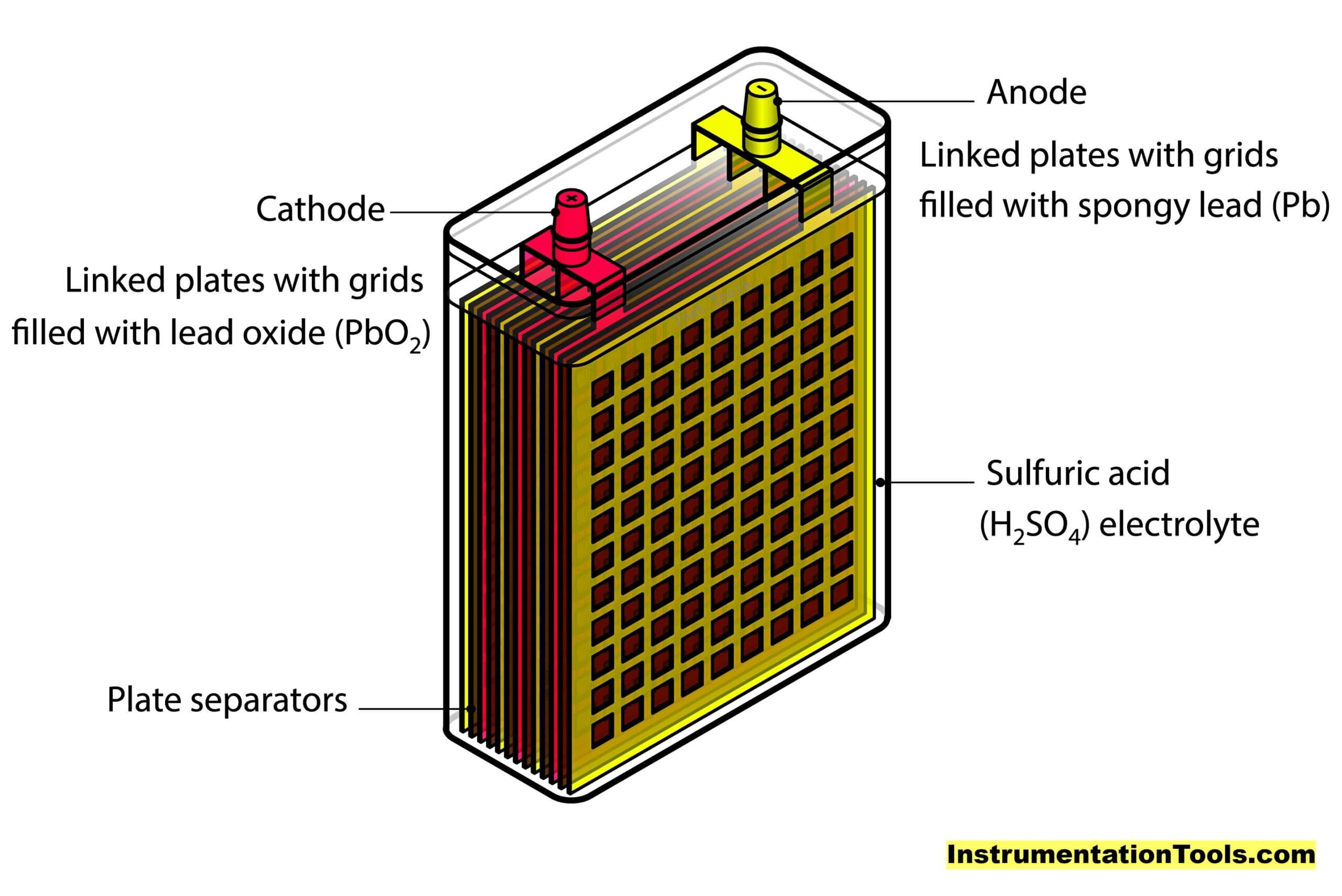
Source: Inst Tools