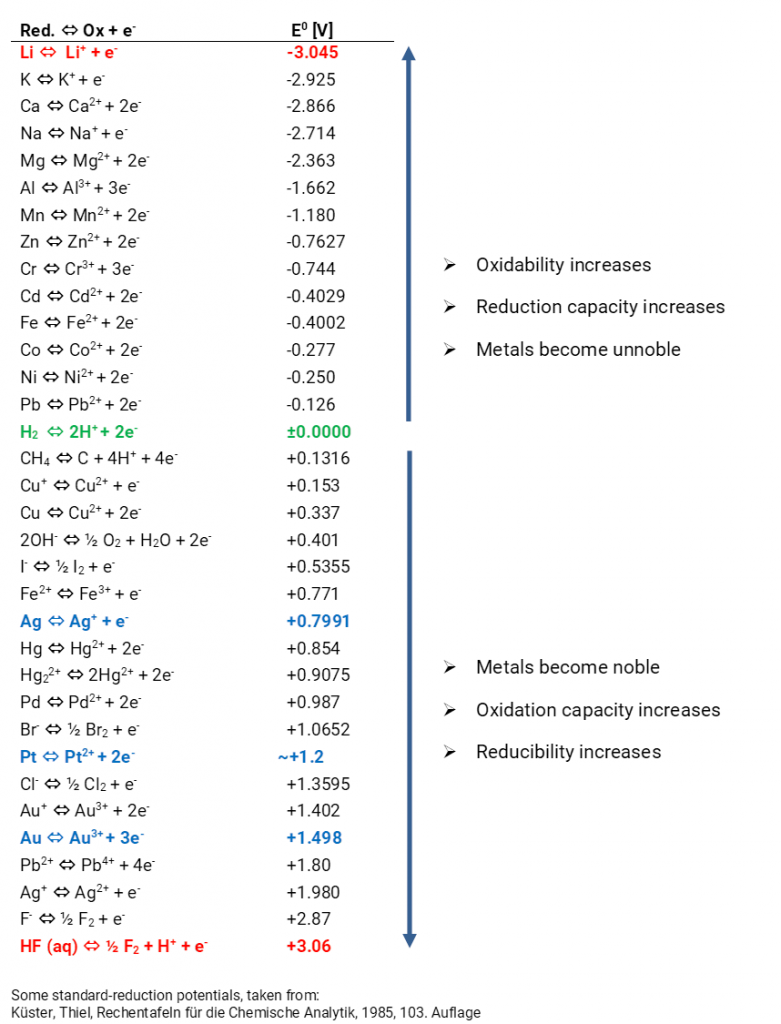
Source: Gaskatel
### Understanding Electrochemical Potential in Batteries
The electrochemical potential difference between an oxidation and reduction reaction in a battery is crucial for its functioning. This potential arises from the differing electrochemical potentials of the reduction and oxidation reactions involved.
#### Electrochemical Potential Explained
The electrochemical potential is a measure of the difference in energy between the outermost electrons of a molecule or element in its two valence states. This potential can be understood by examining the electronic configuration of the material’s outer shell, which indicates the magnitude and sign of the electrochemical potential between reactants and products in a redox reaction.
#### Energy Considerations in Reactions
For materials, the lowest energy configuration is when the outer shell is fully occupied by electrons. When an element loses electrons, it transitions to a stable, low-energy state. Conversely, gaining electrons increases the energy level. This energy difference is reflected in the electrochemical potential of the reaction.
#### Mnemonic Devices for Understanding Reactions
To remember the difference between oxidation and reduction reactions, mnemonic devices can be helpful. For instance, “LEO (the Lion goes) GER (grrr)” signifies “Loss of Electrons is Oxidation” and “Gain of Electrons is Reduction.” Mnemonics like “OIL RIG” (Oxidation Involves Loss, Reduction Involves Gain) can also aid in distinguishing between the two processes.
#### Anode and Cathode in Batteries
Understanding which reactions occur at the anode (oxidation) and cathode (reduction) is essential. Mnemonics like “RED CAT and AN OX” or noting the order of vowels and consonants in “anode” and “cathode” can help in recalling this information. Additionally, remembering that the anode is negative and the cathode is positive is crucial for comprehending battery operations.
#### Conclusion
Electrochemical potential plays a vital role in battery reactions, influencing the flow of electrons and energy transfer. By grasping the concepts of oxidation, reduction, anode, and cathode, one can better understand the inner workings of batteries and their applications in various technologies.
Source: ACS Publications – American Chemical Society